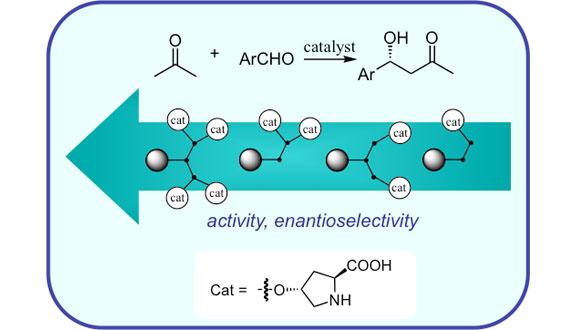
Catalysis
Acceleration of a chemical transformation by a compound that is, formally, not a part of the reaction equation, commonly known as catalysis, is among the most important phenomena in chemistry.
Catalysis is an ideal solution to an ever-growing pressure on the chemical industries to perform reactions in a more efficient, selective and environmentally benign manner.
Optimal catalytic systems enable to perform the reactions under milder conditions, reduce or eliminate generations of hazardous waste, shorten the times for making target products from starting material, and perform transformations unattainable by standard stoichiometric methods. For these reasons, studies of various aspects of catalysis, among them homogeneous and heterogenous catalysis, chemo- and stereo-selective catalysis, biocatalysis, as well as the investigation of catalytic mechanisms, are among the "hottest", most active and dynamic areas of chemical research.
Researchers:
Prof. Dobrovetsky Roman, Dr. Kaminker Ilia, Prof. Kol Moshe, Prof. Portnoy Moshe, Prof. Shabat Doron, Prof. Vigalok Arkadi.